Some worms can even grow their heads – brains and all – back after amputation. Fluorescent worms now give us a glimpse into this remarkable process.
Some worms are capable of bizarre things. For example, if you cut the head of the sea worm Hofstenia miamia cuts it off, a new one simply grows, including an entirely new brain. Do you cut off his tail; same. Do you cut the worm into three separate pieces? Within eight weeks, three fully formed specimens are crawling before your eyes. “These worms are phenomenal at regenerating,” said researcher Mansi Srivastava in conversation with Scientias.nl. Reason enough for scientists to study the Tic Tac-sized worm and learn how to pull off this amazing feat.
Hofstenia miamia
It should be clear: the sea worm Hofstenia miamia is king at regenerating body parts. “They can regrow any cell type that they lose through injury,” Srivastava says. Wonderful and therefore very interesting. Because exactly how these worms achieve this is not yet entirely clear. So researchers decided to subject the critters to a thorough inspection. “Understanding how regeneration works is important for a full understanding of animal biology,” explains Srivastava. “In addition, by studying it, we can find out whether there may also be shared molecular and cellular properties that are essential for regeneration. It is important to understand how regeneration works in species that are good at it before we start solving the problems of human regeneration.”
Glow in the dark
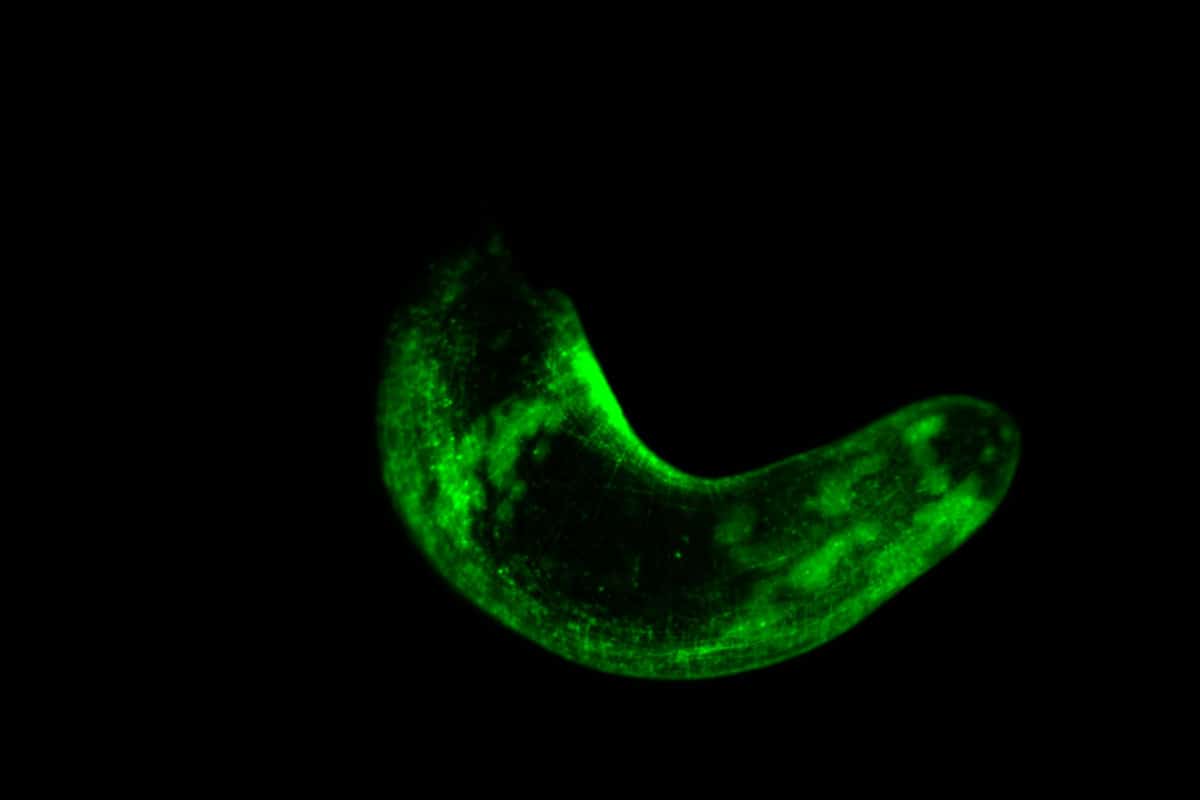
However, the researchers decided not to just put the critters under a microscope. In their studies they created real glow in the dark worms; worms that glow in the dark. The scientific way of saying this is that the worms have now become ‘transgenic’. Transgenesis is when scientists introduce something into an organism’s genome that is not normally part of that genome. “It’s a tool that biologists use to study how cells or tissues work in an animal’s body,” explains Srivastava. The researchers gave the worms a gene that, when it becomes a protein, gives off a certain fluorescent glow. These fluorescent proteins glow green or red and can, for example, lead to glowing muscle and skin cells.

One of the manufactured glow in the dark worms. The muscle cells light up green here. Image: Lorenzo Ricci
Then what makes this fluorescent glow possible is the ability to visualize in much greater detail what cells look like, where they are in the animal, and how they interact with each other. In short, it allows researchers to study the specific mechanism of each process in an organism. And so is regeneration. For example, the fluorescent worms allowed the research team to study in unprecedented detail a group of stem cells that are crucial for regeneration. “We don’t actually know how cells behave in an animal during this process,” Srivastava says. “But thanks to the transgenic worms, we can see the cells in the context of the animal as it regenerates.”
New insights
Thanks to glow in the dark worms, researchers have now gained interesting new biological insights into how these remarkable creatures can regrow amputated limbs. “The transgenic worms show us that muscle cells connect to each other and to other cells – such as those in the skin and gut – via finger-like projections,” Srivastava explains when asked. The researchers saw that muscle cells have a kind of ‘spur’ that interlock in tight columns, forming a tight grid that gives the worm structure and support, almost like a skeleton. “Previous studies had suggested that muscle is important for proper regeneration in worms,” Srivastava continues. “And now we think that the studied compounds play a crucial role in this. It can hold tissues together and/or send signals through these protrusions.”
Follow-up research
The team hopes to find out in follow-up research whether the muscles not only hold parts together, but also store and communicate information about what needs to be regenerated. And we may soon be able to count on new results. It takes about eight weeks to create transgenic worms. Modified DNA is injected into embryos that have just been fertilized. That DNA and the modifications are then incorporated into the genome of the cells as they divide. And as the worm grows, it will glow in the dark. That fluorescent glow will then be passed on to subsequent offspring. And by studying these worms, the researchers hope to gain further insights into how regeneration works.
Meanwhile, the researchers have grown quite attached to the remarkable marine worms. “They are absolutely charming,” said Srivastava. Not least because they sometimes exhibit particularly intriguing behavior and can even be voracious cannibals. For example, if they are not fed for a while and there are several together in one tank, they sometimes dare to take a bite from each other. Regeneration then comes in particularly handy. Future research will hopefully provide further answers to the question of how exactly they do this and why. And who knows, we humans may ultimately benefit from it.
Source material:
“What can scientists learn from worms that glow in the dark? The secrets of regeneration for starters– Harvard University (via EurekAlert)
Interview with Mansi Srivastava
Image at the top of this article: Lorenzo Ricci