
American scientists have discovered a new way in which water can freeze.
Water ice is water ice, you might say. Okay, you have rockets, pear ice creams and so on. But if you freeze nothing but pure water—that is, molecules made up of one oxygen atom and two hydrogen atoms—you simply get ice—right?
Well no. Nineteen ‘main types’ of ice are now known, numbered with Roman numbers: from ice I (the ice we know) to the last year confirmed ice XIX† By pressing water together between two diamonds, physicist Zach Grande of the University of Nevada Las Vegas and his team have now created a managed to make a new kind of ice cream: not ice XX, but ice VIIt.
quadrangular ice cream
As the name implies, ice VIIt resembles ice VII. Ice VII is formed at a pressure of 2.1 gigapascals, in other words: a pressure more than 20,000 times higher than that at the earth’s surface. In this form of ice, the oxygen atoms from the water are arranged like cubes, with one oxygen atom in the center of the cube and one at each corner.
Now if you increase the pressure further, to about 5 gigapascals, those cubes turn into beams, Grande and colleagues found. Because the resulting shape of ice is not different enough from ice VII to give it a whole new Roman number, they named this ice type VIIt. The t stands for tetragonal (‘quadriangular’).
Much lower pressure
Now this new form of ice cream is not a terminal. If you press the diamonds in between the ice even harder, you get ice X. In this form of ice, the hydrogen atoms are exactly between the oxygen atoms.
It was already known that ice VII changes into ice X at some point. However, researchers thought – based on both theoretical calculations and previous experiments – that this only happens at a pressure of about 100 gigapascals. Grande’s work now shows that ice X is already formed at a pressure of 30 gigapascals.
Small grains
Where does that big difference come from? “Theoretical calculations that predict the pressure at which ice X forms are largely based on classical physics,” explains Grande. “Effects from quantum mechanics (the theory that describes the world of the smallest – ed.) are ignored. If you do take it with you, you end up with a much lower pressure.”
And those experiments in which ice X only formed at a much higher pressure? In addition, different parts of the ice experienced different pressures, Grande says, which influenced the measurements. To prevent that, the team he was part of used a laser that divided the ice into small grains. As a result, the pressure experienced by each bit of ice was more or less equal and there was less tension on the ice. That made it easier to determine the point at which the ice transitioned to ice X.
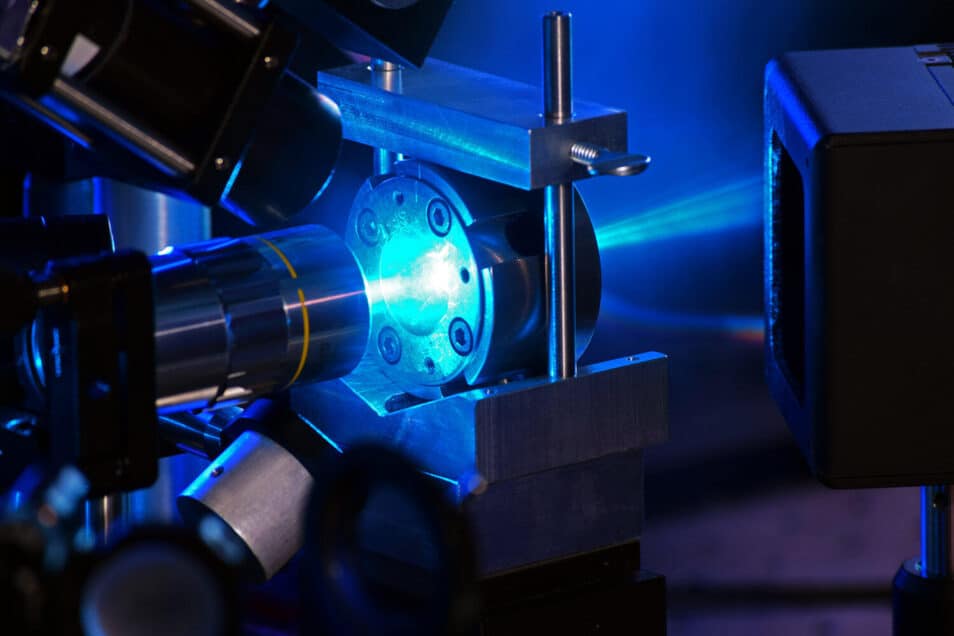
Water is put under high pressure by two diamonds, while a laser shines on it, which breaks up the resulting ice into small particles. (Photo: Chris Higgins/UNLV)
Thin layer of ice
The result has consequences for how we think about planets elsewhere in the universe that consist largely of water. According to the old view, a good part of that water should have been in the form of ice VII, which would have turned into ice X at great depths (where the pressure is very high). think of a fairly thin layer of ice VII, underneath a thick layer of ice VIIt, and soon ice X underneath. “Although in reality it will be more complicated,” says Grande. “In our experiment, we used pure water, while planets contain a lot of different substances.”
Furthermore, the work of Grande and colleagues shows that ice X is much less easily compressed than previously thought. “That means planets that contain a lot of water will be larger than we previously assumed.”
Source material:
†Pressure-driven symmetry transitions in dense H2oh ice” – Physical Review B
†UNLV Physicists Discover New Form of Ice” – University of Nevada, Las Vegas
Zachary Grande (University of Nevada, Las Vegas) Image at the top of this article: goumbik through Pixabay