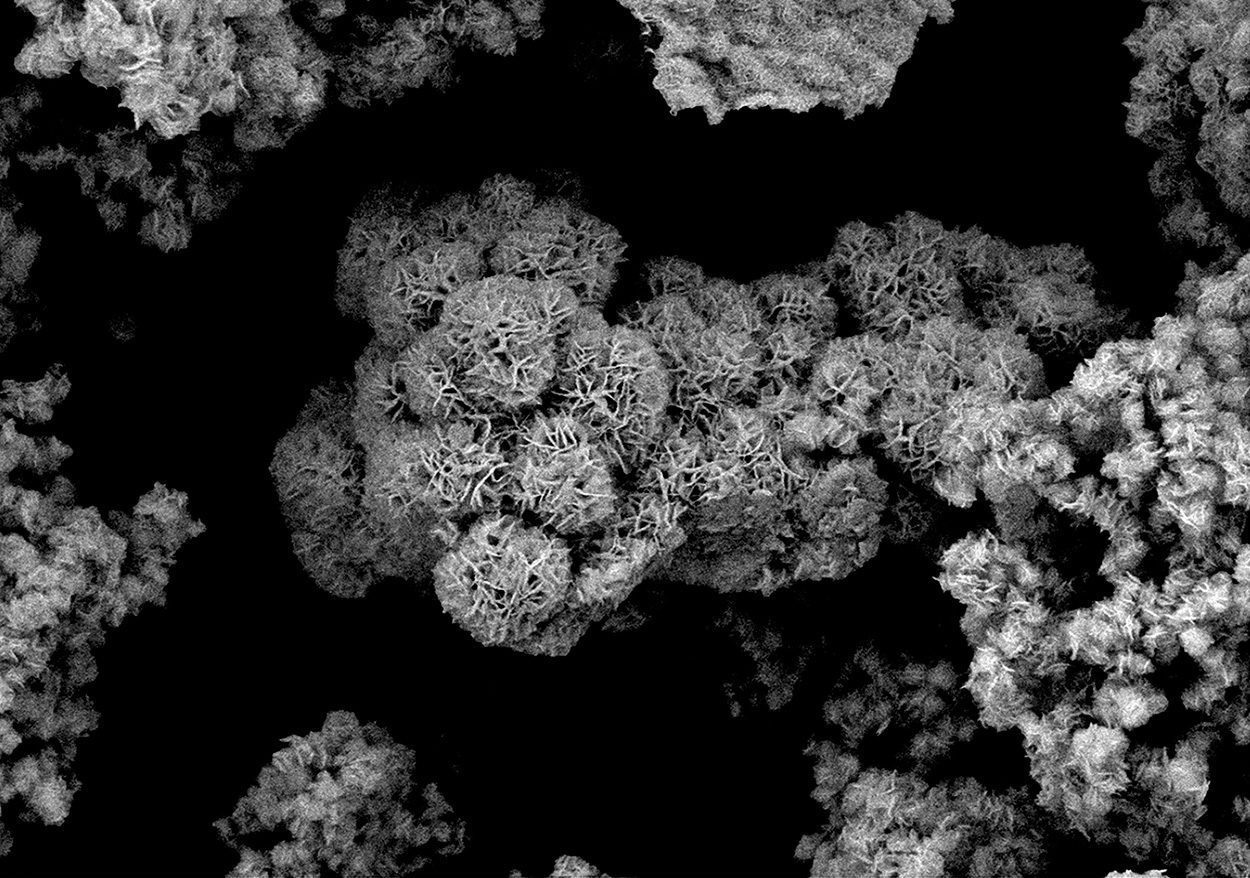
These porous, branched-out spheres are made of molybdenum sulfide and are part of a new catalyst that can convert carbon dioxide into methanol.
In order to advance climate protection, no more climate-damaging carbon dioxide (CO2) should be allowed into the atmosphere. One way to curb CO2 emissions is to capture the greenhouse gas from the exhaust stream of industrial plants or power plants. Ideally, the separated CO2 is then bound in other, non-volatile materials or reused. One way to do this is to chemically convert the gas into methanol, a liquid that can be used as a fuel and chemical raw material.
“Until now, copper-based catalysts have often been used to convert carbon dioxide,” explains Karin Föttinger from the Institute of Materials Chemistry at the Vienna University of Technology. “However, they have the major disadvantage that they are not robust. If, in addition to carbon dioxide, certain other substances are also present in the exhaust gas stream, such as sulfur, the catalytic converter quickly loses its effectiveness. They say the catalyst is poisoned.” Föttinger and her team therefore looked for catalyst alternatives that are less susceptible to this chemical degradation.
“If you want to use such methods not only in the laboratory, but also on a large scale in industry, then you need a catalyst that is perhaps a little less active, but robust, durable and reliable,” says Föttinger. “One would like to be able to process normal industrial exhaust gases without pre-treatment.” Through their research, the scientists discovered that compounds of molybdenum and sulfur are suitable for the catalysis of CO2 to form methanol.
The molybdenum sulfide shown in our picture in an electron micrograph forms porous, spherical microstructures that trap the gas and provide plenty of space for chemical reactions thanks to their large surface area. Special additives such as manganese facilitate the splitting and conversion of the actually rather unreactive carbon dioxide. “If you want, you can also use our catalysts to produce other molecules, such as higher alcohols,” explains Föttinger. “We are currently still working on finding out exactly how parameters such as pressure and temperature are best selected in order to produce different products.” The new technology has already been patented, and together with industrial partners the process is now to be scaled up to an industrial scale.